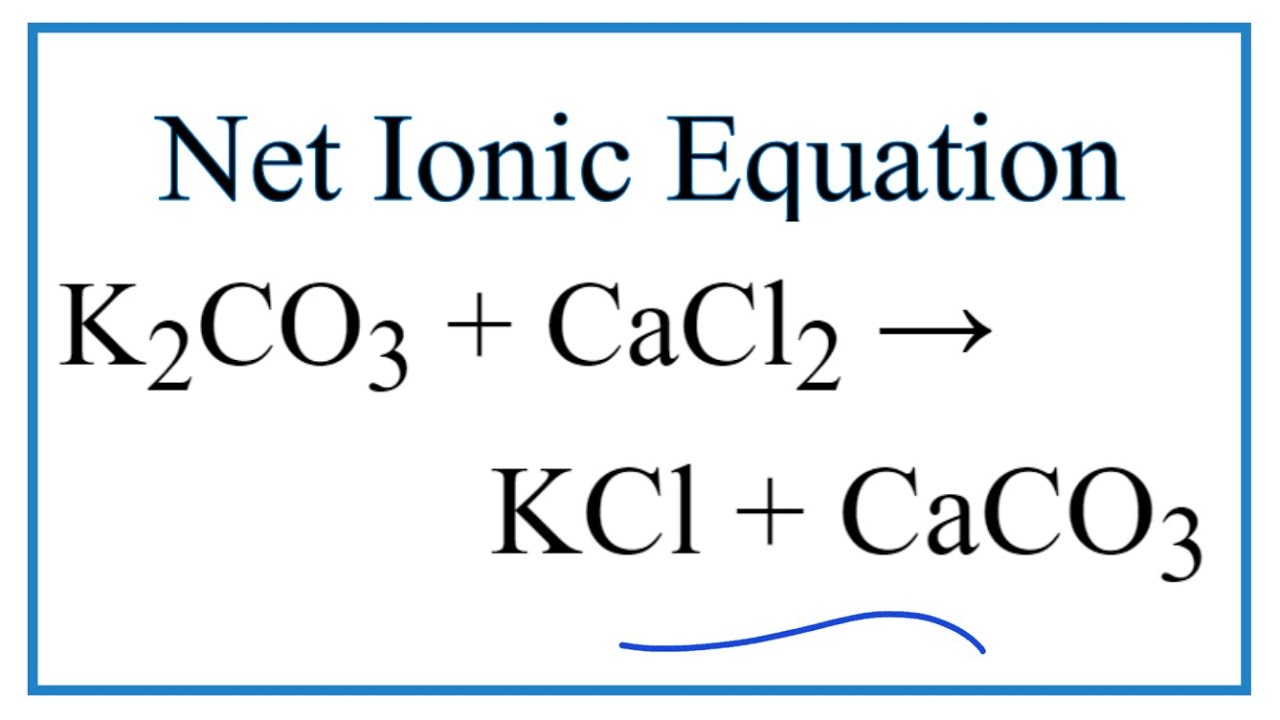Chemistry is a fascinating field that opens up a world of possibilities, especially when it comes to understanding chemical reactions and their implications. One such intriguing interaction is between calcium chloride (CaCl2) and potassium carbonate (K2CO3). This reaction not only showcases the beauty of chemical transformations but also highlights the importance of these compounds in various applications, from industry to agriculture. As we delve deeper into the chemistry of CaCl2 and K2CO3, we’ll uncover the mechanisms behind their reactions and the significance of their products.
Understanding the chemistry behind CaCl2 + K2CO3 is essential for students, researchers, and industry professionals alike. The reaction between these two compounds leads to the formation of calcium carbonate (CaCO3) and potassium chloride (KCl), which are both valuable substances used in multiple fields. By exploring the properties and behavior of these chemicals, we can appreciate their utility and the broader implications of their reactions in real-world applications.
This article aims to provide a comprehensive overview of the reaction between CaCl2 and K2CO3. We will explore the underlying principles, the products of the reaction, and their applications in various industries, including agriculture, water treatment, and food production. Join us as we embark on this chemical journey to unveil the secrets of CaCl2 + K2CO3 and discover how these compounds interact to create valuable products.
What is CaCl2 and K2CO3?
Calcium chloride (CaCl2) is an inorganic salt that is highly soluble in water. It is commonly used for de-icing roads, as a desiccant, and in various industrial processes. Potassium carbonate (K2CO3), on the other hand, is another inorganic compound that serves as a source of potassium, essential for plant growth and various industrial applications.
How do CaCl2 and K2CO3 React Together?
When calcium chloride is mixed with potassium carbonate, a double displacement reaction occurs. The calcium ions (Ca²⁺) from CaCl2 react with the carbonate ions (CO3²⁻) from K2CO3 to form calcium carbonate (CaCO3), while the potassium ions (K⁺) combine with the chloride ions (Cl⁻) to produce potassium chloride (KCl).
What are the Products of the Reaction Between CaCl2 and K2CO3?
- Calcium Carbonate (CaCO3): A sparingly soluble compound used in various applications, including as a dietary supplement and in construction.
- Potassium Chloride (KCl): A soluble salt used in fertilizers and food processing.
What is the Chemical Equation for the Reaction?
The chemical equation for the reaction between CaCl2 and K2CO3 can be represented as follows:
CaCl2 + K2CO3 → CaCO3 + 2 KCl
What are the Applications of the Products?
The products of the reaction, calcium carbonate and potassium chloride, have wide-ranging applications:
- Calcium Carbonate: Used in the production of cement, as a filler in plastics, and as a dietary supplement.
- Potassium Chloride: Employed in fertilizers, as a salt substitute in food, and in medical treatments.
How is This Reaction Relevant in Agriculture?
The reaction between CaCl2 and K2CO3 plays a significant role in agriculture. The resulting potassium chloride serves as a key nutrient source for crops, while calcium carbonate contributes to soil health and pH regulation. Farmers and agricultural scientists often utilize these compounds to enhance crop yields and improve soil quality.
What Safety Precautions Should Be Taken When Handling CaCl2 and K2CO3?
When working with chemicals like CaCl2 and K2CO3, it's essential to observe safety precautions:
- Wear protective gloves and goggles.
- Work in a well-ventilated area.
- Store chemicals in a cool, dry place away from incompatible substances.
Conclusion: The Significance of Understanding CaCl2 + K2CO3
In conclusion, the reaction between calcium chloride and potassium carbonate is an excellent example of chemical processes that have significant implications in various fields. From agriculture to industrial applications, the products of this reaction—calcium carbonate and potassium chloride—play crucial roles in our daily lives. By understanding the chemistry behind CaCl2 + K2CO3, we can appreciate the importance of these compounds and their contributions to our world.
Article Recommendations
- New Year Movie 2024
- Dianna Williams Bring It
- Finalists On Dancing With The Stars
- Nikocado Avocado Fit
- Cast From The Secret Life Of The American Teenager
- How Old Is Helena Vestergaard
- Gunsmoke Lijah
- Matilda Today
- Going Gluten Free Changed My Life
- How Old Is Brielle From Ellen