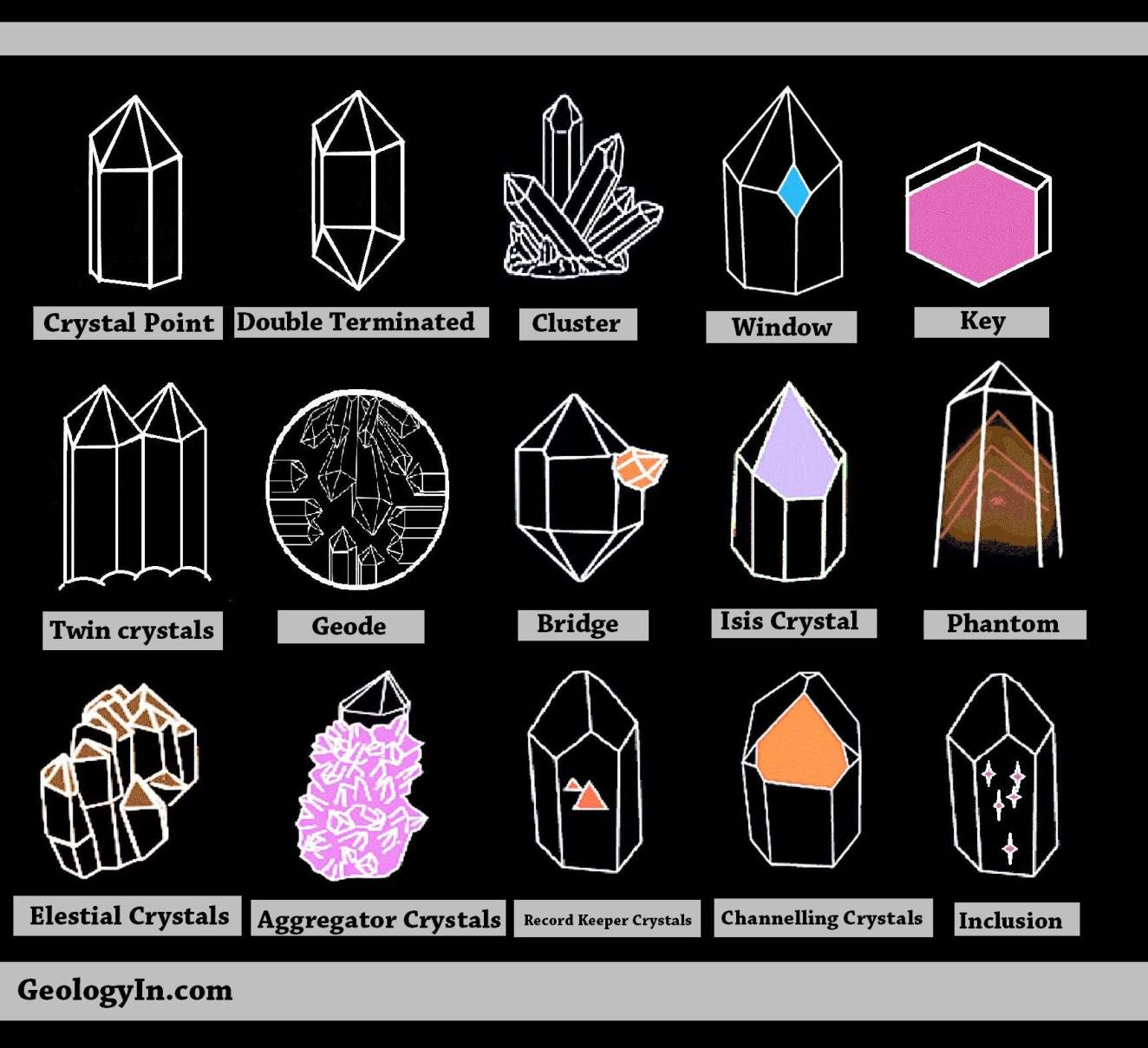
Crystals have fascinated humanity for centuries, not just for their beauty but also for their unique structures and properties. Understanding different crystal shapes can enhance our appreciation of these natural wonders and their applications in various fields. In this article, we will delve into the various shapes of crystals, their significance, and how they are formed. By the end of this guide, you will have a deeper understanding of the diverse world of crystals and how they impact our lives.
Crystals can be found in many forms, from the sparkling gemstones adorning jewelry to the simple salt on your kitchen table. Their shapes are determined by the arrangement of atoms within the crystal lattice, leading to a variety of stunning geometrical forms. As we explore the different crystal shapes, we'll also touch on their properties and uses in industries ranging from electronics to healing practices.
Whether you are a gem enthusiast, a scientific researcher, or simply curious about the natural world, this article will provide you with valuable insights into the fascinating realm of crystal shapes. Let's embark on this journey of exploration and discover the beauty and complexity of crystals together.
Table of Contents
- What Are Crystals?
- Importance of Crystal Shapes
- Types of Crystal Structures
- Crystal Growth Process
- Applications of Crystals
- Healing Properties of Crystals
- Conclusion
What Are Crystals?
Crystals are solid materials whose atoms are arranged in a highly ordered and repeating pattern, known as a crystal lattice. This unique arrangement gives crystals their distinct shapes and properties. Crystals can form through various processes, including cooling from a molten state, evaporating from a solution, or through biological processes.
Some common examples of crystals include:
- Quartz
- Diamond
- Salt (Sodium Chloride)
- Ice
The study of crystals is known as crystallography, a branch of science that examines the arrangement of atoms within crystals and their interactions. Understanding the structure of crystals is crucial in fields such as chemistry, geology, and materials science.
Importance of Crystal Shapes
The shape of a crystal significantly influences its physical and chemical properties. Different crystal shapes can affect how light interacts with the crystal, its hardness, and its conductivity. For example, the cubic shape of diamond contributes to its brilliance and hardness, making it one of the most sought-after gemstones.
Moreover, the symmetry and geometry of crystals play a crucial role in determining their behavior in various applications:
- Optical Properties: The shape of a crystal can affect its refractive index, which in turn influences how it bends and transmits light.
- Mechanical Properties: The arrangement of atoms in a crystal shape can determine its hardness and brittleness.
- Chemical Reactivity: Different crystal shapes can interact differently with other substances, affecting their reactivity.
Types of Crystal Structures
Crystals can be classified into several types based on their shapes and symmetry. Here are the four primary crystal structures:
Cubic Crystals
Cubic crystals are characterized by their three axes of equal length, intersecting at right angles. This structure is commonly seen in minerals such as:
- Table salt (sodium chloride)
- Cubic zirconia
- Pyrite
The regularity of the cubic structure allows for a uniform distribution of stress, making these crystals particularly strong.
Hexagonal Crystals
Hexagonal crystals feature a six-sided symmetry with three axes of equal length and one axis of a different length. Common examples include:
- Quartz
- Graphite
- Tourmaline
The hexagonal structure often leads to unique optical properties, making these crystals popular in jewelry and decorative items.
Tetragonal Crystals
Tetragonal crystals have two axes of equal length and one that is different, forming a rectangular prism shape. Examples include:
- Rutile
- Vermiculite
The tetragonal structure can influence the crystal's electrical and magnetic properties.
Orthorhombic Crystals
Orthorhombic crystals feature three axes of unequal lengths that intersect at right angles. Common examples are:
- Topaz
- Barite
The irregularity in the axes can lead to varying physical properties, making orthorhombic crystals interesting for both collectors and scientists.
Crystal Growth Process
The formation of crystals occurs through a process known as crystallization. This process can take place through several methods, including:
- Cooling: As a liquid cools, the atoms or molecules begin to bond and form a solid structure.
- Evaporation: When a solution containing dissolved minerals evaporates, the remaining particles can bond together to form crystals.
- Biological Processes: Some organisms can produce crystals as part of their metabolic processes, such as the formation of shells in mollusks.
Each of these processes can lead to different crystal shapes and sizes, influenced by factors such as temperature, pressure, and the concentration of the solution.
Applications of Crystals
Crystals have a wide range of applications across different industries, including:
- Electronics: Semiconductors and other electronic components often rely on specific crystal structures for optimal performance.
- Jewelry: Many gemstones are prized for their beauty and rarity, as well as their unique crystal shapes.
- Medicine: Some crystals are used in medical applications, such as X-ray crystallography for analyzing the structure of biological molecules.
Healing Properties of Crystals
In addition to their physical applications, many believe that crystals possess healing properties. Different crystal shapes are thought to interact with energy fields, potentially promoting healing and well-being. Some popular crystals used for healing include:
- Amethyst: Known for its calming effects and ability to promote emotional balance.
- Rose Quartz: Often associated with love and compassion.
- Citrine: Believed to promote abundance and positive energy.
While scientific evidence may be limited, many individuals report positive experiences when using crystals for healing and meditation.
Conclusion
In summary, the world of crystal shapes is both fascinating and diverse. From cubic to hexagonal structures, each crystal form holds unique properties that impact its use in various fields. Understanding different crystal shapes not only enhances our appreciation of these natural wonders but also highlights their significance in science and daily life.
We encourage you to explore the world of crystals further. Consider adding some beautiful specimens to your collection or researching their potential healing properties. If you have any thoughts or experiences with crystals, feel free to leave a comment below!
Penutup
Thank you for reading! We hope this article has provided you with valuable insights into different crystal shapes. Stay curious and continue exploring the wonders of nature. We look forward to seeing you back here for more informative articles!
Article Recommendations
- Who Is Alan Greenspan
- Chantel And Pedro
- Robert James Ritchie Jr
- New Year Movie 2024
- Great British Bake Off Shop
- Neil Young Images
- Rod Steart Age
- Nikocado Avocado Fit
- Finalists On Dancing With The Stars
- Aj And Kandi Burruss
/GettyImages-583676186-58a1fe0a3df78c47586a758d.jpg)

