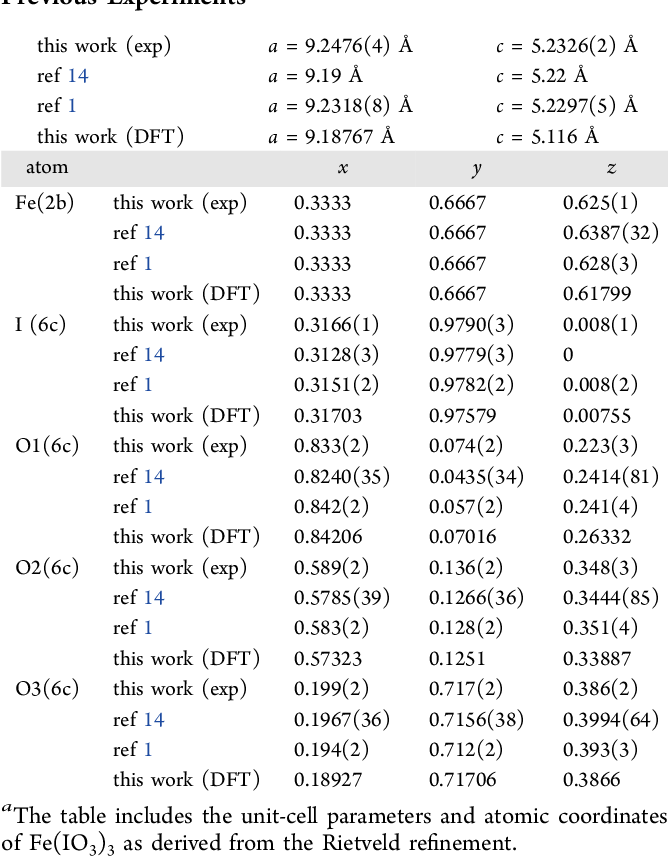
The compound Fe(IO3)3, also known as Iron(III) iodate, is a fascinating chemical that plays a significant role in various scientific applications. This unique compound is formed by the combination of iron ions and iodate ions, leading to intriguing properties that are essential in the fields of chemistry and materials science. Iron(III) iodate is often utilized in laboratory settings and has implications in environmental studies, making it a compound worth exploring in-depth.
The study of Fe(IO3)3 not only provides insight into its chemical structure but also showcases its applications in different industries. As we delve into the characteristics and significance of Iron(III) iodate, we will uncover its uses, formation, and how it compares to similar compounds. Understanding the Fe(IO3)3 compound name is crucial for anyone interested in chemistry, as it opens the door to a broader comprehension of compounds containing iodine and iron.
In this article, we will address various aspects of the Fe(IO3)3 compound, including its nomenclature, properties, and potential applications. By answering essential questions and presenting detailed information, we aim to provide a comprehensive guide to the Iron(III) iodate compound that will be valuable for students, researchers, and enthusiasts alike.
What is the Chemical Structure of Fe(IO3)3?
The chemical structure of Fe(IO3)3 is based on the presence of iron in the +3 oxidation state, bonded to three iodate ions (IO3-). Each iodate ion consists of one iodine atom surrounded by three oxygen atoms arranged in a trigonal planar configuration. The overall structure can be represented as follows:
- Fe: Iron in +3 oxidation state
- IO3: Iodate ion
How is Fe(IO3)3 Formed?
The formation of Fe(IO3)3 typically occurs through a reaction between iron(III) salts and iodic acid (HIO3). The reaction can be represented by the following equation:
FeCl3 + 3 HIO3 → Fe(IO3)3 + 3 HCl
This reaction highlights the importance of the reactants and the conditions under which Iron(III) iodate can be synthesized.
What Are the Properties of Fe(IO3)3?
The properties of Fe(IO3)3 make it a unique compound with various applications:
- Appearance: Fe(IO3)3 typically appears as a yellowish solid.
- Solubility: It is moderately soluble in water, which is a vital consideration for its use in solutions.
- Stability: The compound is stable under normal conditions but can decompose when exposed to high temperatures.
What Are the Applications of Fe(IO3)3?
Fe(IO3)3 has several applications across various fields:
- Chemical Analysis: It is used as a reagent in analytical chemistry.
- Environmental Studies: The compound can be utilized in the study of iodine compounds in environmental samples.
- Research: Fe(IO3)3 is used in various research studies related to inorganic chemistry.
How Does Fe(IO3)3 Compare to Other Iodates?
When comparing Fe(IO3)3 to other iodates, such as potassium iodate (KIO3) or sodium iodate (NaIO3), some differences become evident:
- Solubility: KIO3 and NaIO3 are more soluble in water than Fe(IO3)3.
- Oxidation States: The oxidation states of the metal ions differ, influencing their chemical properties.
- Reactivity: The reactivity of Fe(IO3)3 can also differ, especially in redox reactions.
Is Fe(IO3)3 Safe to Handle?
Handling Fe(IO3)3 requires standard laboratory safety precautions, as with any chemical compound. Protective equipment such as gloves, goggles, and lab coats should be worn to prevent exposure. It is essential to follow safety guidelines when working with this compound to ensure a safe laboratory environment.
Conclusion: Why is Understanding Fe(IO3)3 Important?
Understanding the Fe(IO3)3 compound name and its properties is crucial for anyone engaged in scientific research or education. With its applications ranging from chemical analysis to environmental studies, this compound exemplifies the fascinating intersection of chemistry and practical applications. As we continue to explore compounds like Iron(III) iodate, we deepen our understanding of the fundamental principles that govern chemical interactions and their implications in the real world.
Article Recommendations
- Liza Weil
- How Old Is Brielle From Ellen
- Who Is Felicity In Arrow
- Going Gluten Free Changed My Life
- What Team Does Anthony Rizzo Play For
- Trent Williams Tattoos
- How Many Seasons Is Steve On Shameless
- Glitter And Gold Theme Party
- Yk Osiris And Diddy
- Ribcage Straight Ankle Jeans