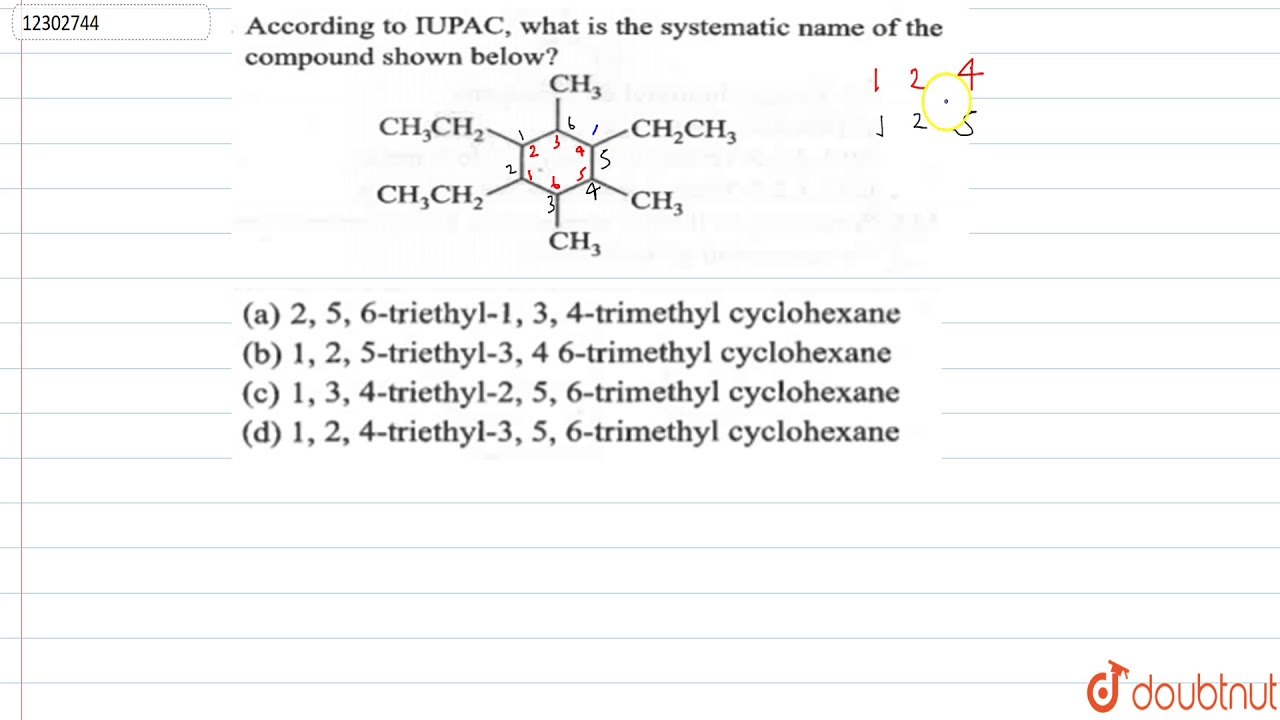
In the realm of chemistry, precise communication is paramount. The systematic naming of compounds allows scientists to convey complex information in a straightforward manner. When we encounter a compound such as CO2 SO4 3, it raises essential questions about its structure, composition, and the appropriate name that encapsulates its essence. Understanding how to derive the systematic name for the compound CO2 SO4 3 is not just an academic exercise but a critical skill in the scientific community.
Systematic names are derived from the rules outlined by the International Union of Pure and Applied Chemistry (IUPAC), which provides a standardized approach to naming chemical substances. This ensures that chemists around the world can accurately identify and discuss compounds without ambiguity. In the case of CO2 SO4 3, we are presented with a unique combination of elements that necessitates careful consideration to arrive at the correct systematic name.
As we delve deeper into the naming conventions and the chemistry involved in CO2 SO4 3, we will explore its molecular structure, the significance of its components, and how to systematically name such a compound. This journey not only enhances our understanding of chemical nomenclature but also underscores the importance of clarity and precision in science.
What Does CO2 SO4 3 Represent in Chemistry?
CO2 SO4 3 is a chemical formula that suggests a compound containing carbon dioxide (CO2) and sulfate (SO4). However, it is important to note that the way this compound is presented lacks clarity according to standard chemical notation. To understand its true nature, we need to break down the components of this formula.
How is CO2 SO4 3 Structured?
To grasp the systematic name for the compound CO2 SO4 3, we must first examine its structure. The compound appears to be a combination of carbon (C), oxygen (O), and sulfur (S) atoms, with the following components:
- Carbon Dioxide (CO2): Comprising one carbon atom and two oxygen atoms.
- Sulfate (SO4): Consisting of one sulfur atom and four oxygen atoms.
So, what we have here is a compound that may imply the presence of carbonate and sulfate functionalities. However, the question remains: is there a valid way to combine these two distinct groups in a single systematic name?
Is CO2 SO4 3 a Valid Compound?
To determine whether CO2 SO4 3 can exist as a valid compound, we need to consider the chemical properties and bonding characteristics of carbon dioxide and sulfate. While both components are indeed stable in their respective forms, combining them directly into a single compound raises questions about their interactions and whether such a structure can maintain stability.
What is the Systematic Name for CO2 SO4 3?
The systematic name for CO2 SO4 3 can be rather complex due to the nature of the components involved. Instead of directly naming the compound as presented, chemists often resort to naming the individual components and their respective roles within the compound. In this case, the most appropriate systematic name would be “Carbon Sulfate” or “Carbon Dioxide Sulfate.”
What are Alternative Naming Conventions?
In addition to the systematic name, there are alternative naming conventions that chemists might use to describe the compound. Here are some possibilities:
- Carbonate Sulfate
- Sulfated Carbon Dioxide
These names highlight the presence of both carbon and sulfate functional groups while adhering to the conventions of chemical nomenclature.
What Are the Applications of CO2 SO4 3?
Understanding the applications of a compound like CO2 SO4 3 can provide context for its systematic naming. While this specific compound may not be prevalent in conventional chemistry, the individual components—carbon dioxide and sulfate—are widely used in various industries:
- Carbon Dioxide: Used in carbonated beverages, refrigeration, and as a raw material in chemical syntheses.
- Sulfate: Commonly employed in fertilizers, detergents, and in the production of sulfuric acid.
Why Is Systematic Naming Important in Chemistry?
The importance of systematic naming in chemistry cannot be overstated. It serves several key purposes:
- Ensures clarity and eliminates ambiguity in communication among scientists.
- Facilitates the understanding of chemical structures and their properties.
- Enables the systematic classification and organization of compounds.
What Are the Challenges in Systematic Naming?
Despite its importance, systematic naming poses challenges, especially with complex compounds. Some of the difficulties include:
- Determining the correct order of naming based on IUPAC rules.
- Understanding the nuances of oxidation states and coordination in multi-element compounds.
- Adapting to the evolving nature of chemistry and new compounds being discovered.
How Can Chemists Improve Their Naming Skills?
To enhance their skills in systematic naming, chemists can take several steps:
- Study IUPAC guidelines and practice naming various compounds.
- Engage in discussions with colleagues and participate in workshops.
- Utilize software tools and databases that assist in compound identification and naming.
In conclusion, the quest to give the systematic name for the compound CO2 SO4 3 unveils a fascinating journey into the world of chemistry. By exploring the components, structure, and naming conventions, we gain a deeper appreciation for the language of science and the importance of clarity in communication. As we continue to navigate the complexities of chemical nomenclature, let us remain committed to precision and understanding in our scientific endeavors.
Article Recommendations
- Understanding My Natal Chart
- Matilda Today
- Laura Von Lindholm Playboy
- De Donde Es La Mama De Donal Trump
- Lee Soo Hyuk Kim Min Hee
- Bob Evans
- Cnn What Does Donal Trump Want To Do With Violence
- How Many Female Governors
- Ryan Paevey Birthday
- How Did Rudolph The Red Nosed Reindeer Originated